As the rapidly evolving coronavirus continues to spread – with the World Health Organization recently confirmed that the virus will remain present for some time – nations are gearing up to monopolize the production of vaccines, giving priority to its people and friendly countries.
This has led the WHO to accuse larger nations of causing a global shortage of vaccines, with developing countries getting the shorter end of the stick.
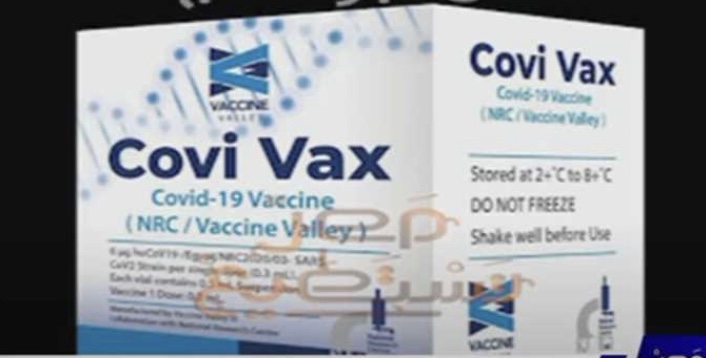
Amid uncertainty about COVID-19 and the emergence of ever more ferocious strains, a ray of hope has come to Egypt after scientists have produced the country’s first locally produced vaccine.
Egypt recently announced the end of laboratory and animal experiments of its new vaccine “Covi Vax” and started first clinical trial on the vaccine, after it was granted approval by the Egyptian Drug Authority.
Al-Masry Al-Youm met with various research teams, which worked on the stages of vaccine production from its inception until conducting clinical trials at the National Research Center.
Up to 70 scientists worked on the vaccine, while biotechnology Egyptian company, Vaccine Valley, is the national partner in the production of the Egyptian vaccine as it equipped special production lines for it.
Al-Masry Al-Youm monitored the team that moved from the lab to the National Research Center to work on clinical trials, which began to receive participating volunteers and conduct full medical examinations to choose the most appropriate among them.
The research team’s role is over and production needs a sovereign decision, said a Professor of Virology at the National Research Center and head of the research team for the Egyptian coronavirus vaccine Mohamed Ahmed Ali.
He explained that the team benefited from the work done on previous experiments, such as swine and bird flu experiments, to produce the vaccine, especially since it has been working on a vaccine for the virus since it appeared in the Chinese city of Wuhan.
“I participated in the clinical trials of the Egyptian vaccine … which I promised my father before his death,” M. Sh., volunteer in the clinical trials of the Egyptian vaccine, said.
“God sent me by chance to be the number one volunteer, as I was at the National Research Center hospital for dental examination. I was surprised by a hanging sign asking for volunteers to the clinical trials for the production of (Covi Vax),” he added.
Egypt celebrates the efforts of a large number of researchers and scientists who defied the circumstances and were able to produce an Egyptian vaccine, Minister of Higher Education and Scientific Research, Khaled Abdel Ghaffar, said.
He extended his thanks to the work team and the workers of the National Research Center.
The head of the clinical trials team for Covi Vax at the National Research Center Osama Azmy confirmed that the first indications of clinical trials that are conducted in three phases, did not detect any problem related to side effects on volunteers.
He added the new vaccine will be available in vaccination centers from six to nine months maximum from now.
Edited translation from Al-Masry Al-Youm