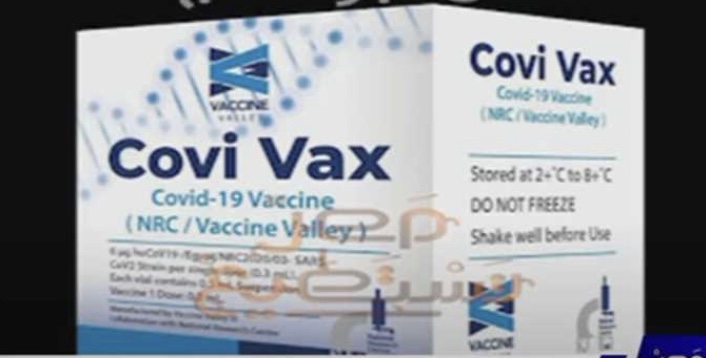
The head of the Clinical Research Unit at Eva Pharma Company Samira Ezzat announced Monday that the new Egyptian coronavirus vaccine “Covi Vax” is awaiting approval by the Egyptian Drug Authority to conduct clinical trials.
During a telephone interview with the “90 Minutes” program on the “al-Mehwar” satellite channel, Ezzat added that “Covi Vax” began its research a year ago, and the experiment trials on animals was completed.
She added that in the first stage, between 30 and 40 all-adult volunteers are subject to clinical trials, explaining that during the second and third stages, the age groups of the participants in the trials can be changed.
The first stage of experiments will not be suitable for children and pregnant women, but will be better in future phases.
She assured it is safe and effective.
Work will be done to develop this vaccine in order to be effective against the Omicron mutant, she said, noting that the target of the vaccine is to cover the Egyptian people, and then export, in support of Egypt’s leadership, in addition to the financial return.
Edited translation from Al-Masry Al-Youm